Prion Disease Treatment: Milestone Achieved by Researchers
Prion disease treatment has emerged as a beacon of hope within the realm of neuroscience research, as scientists unveil groundbreaking findings aimed at combating these currently irreversible and fatal diseases. Among these is Creutzfeldt-Jakob disease, a devastating condition characterized by rapid neurodegeneration caused by misfolded prion proteins. Recent studies underscore the potential of gene-editing therapy, which may significantly reduce the harmful effects of such prion proteins in the brain. Research indicates that modifying the prion protein gene through innovative techniques can extend the lives of affected individuals and improve their quality of life. As patient-scientists like Sonia Vallabh and Eric Minikel champion this cause, the scientific community is inspired to pursue practical treatments that could transform the future of prion disease management.
The exploration of therapies for prion conditions, often referred to as transmissible spongiform encephalopathies, has garnered increased attention as researchers strive to find viable solutions to these intricate and often lethal disorders. These diseases, including fatal familial insomnia, are caused by abnormal folding of proteins within the brain, leading to severe neurodegenerative effects. Recent advancements through innovative methods such as gene-editing approaches hold promise for altering the genetic basis of prion diseases, potentially altering the course of illnesses characterized by extreme neurological decline. The intersection of cutting-edge science and personal narratives brings urgency and determination to the quest for effective interventions against these rare yet critically impactful conditions.
Understanding Prion Diseases: Definition and Types
Prion diseases, also known as transmissible spongiform encephalopathies, are a group of rare and invariably fatal neurodegenerative disorders that affect both humans and animals. These diseases are caused by misfolded prion proteins, which lead to brain damage and cognitive impairment. The most well-known types of prion diseases in humans include Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome, and fatal familial insomnia (FFI). Each of these conditions presents with unique neuropathological features and symptoms, yet they share the common hallmark of abnormal protein aggregation in the brain, ultimately leading to severe neurological decline and death.
The diversity of prion diseases illustrates how variations in the prion protein gene can result in different clinical manifestations. Approximately 15% of prion disease cases are hereditary, stemming from genetic mutations, while the remaining 85% are sporadic, arising from spontaneous misfolding of proteins. Understanding these distinctions is crucial in neuroscience research, as it can guide the development of targeted therapies and genetic interventions suited to specific types of prion diseases.
With advancements in neuroscience research, particularly in gene-editing technology, the hope for effective treatments for prion diseases is brightening. Research is increasingly focusing on the mechanisms of prion propagation and misfolding, which can lead to better insights into disease pathways. Through collaborations like those at the Broad Institute, scientists can share knowledge and techniques to accelerate the discovery of potential therapeutics aimed at halting or even reversing these devastating conditions.
Promising Gene-Editing Therapy for Prion Diseases
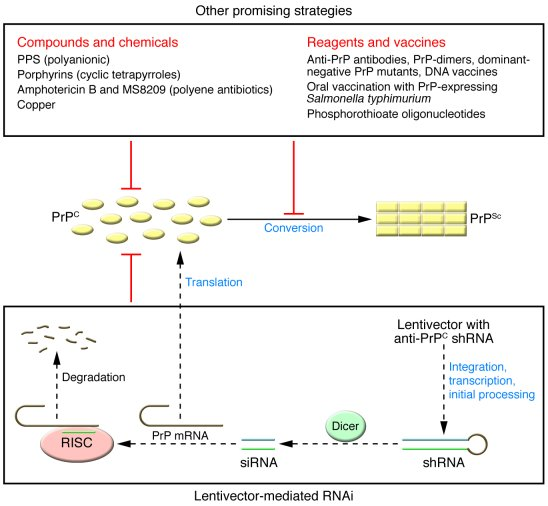
One of the most exciting recent developments in treating prion diseases is the promising gene-editing therapy spearheaded by researchers including Sonia Vallabh and Eric Minikel. This innovative approach, which utilizes a gene-editing technique known as base editing, focuses on correcting a specific mutation in the prion protein gene responsible for producing the harmful prions. The study conducted on laboratory mice showed that altering a single base in the gene resulted in a significant reduction in toxic protein production, thereby improving the lifespan of the mice by 52%. Such remarkable results indicate a potential breakthrough for therapies targeting human prion diseases, highlighting the importance of ongoing research and clinical trials.
Though the research is a significant milestone, it also underscores the complexities of advancing from laboratory findings to human applications. Researchers must navigate a series of rigorous regulatory steps to ensure safety and efficacy before initiating clinical trials. However, the enthusiasm within the scientific community, coupled with the personal stakes faced by researchers like Vallabh, fuels determination to bring this therapy to fruition. The successful application of gene-editing therapy is not only a scientific endeavor but also a deeply personal mission for those directly affected by prion diseases.
In the context of prion disease treatment, the collaborative effort among researchers has proven to be paramount in addressing these complex disorders. The synergy created through partnerships, such as the one between Vallabh, Minikel, and David Liu’s lab, has enabled the sharing of expertise in various areas like vector engineering and disease modeling. By combining knowledge from different fields, scientists can create more effective strategies for targeting prion diseases, increasing the chances of successful outcomes in forthcoming clinical trials. The potential applicability of gene-editing therapy in treating prion diseases offers a glimmer of hope for millions around the world who may be affected by these unfortunate conditions.
The Personal Journey of Patient-Scientists in Research
For many researchers, especially patient-scientists like Sonia Vallabh and Eric Minikel, the journey into scientific research is deeply rooted in personal experience. Vallabh’s diagnosis with fatal familial insomnia (FFI), a hereditary prion disease, catalyzed her and her husband into action—not just for personal reasons, but also to contribute to the broader scientific understanding of these devastating disorders. Their transition from law and urban planning to neuroscience research is a testament to their unwavering commitment to finding a treatment for prion diseases, illustrating how personal narratives can inspire significant advancements in medical science.
Their dedication not only motivates their research but also attracts collaboration from other scientists who share their passion for fighting prion diseases. The emotional weight of their story—having witnessed the decline and death of Vallabh’s mother from FFI—serves as a powerful driving force behind their work. It highlights the profound intersection of personal stakes and professional expertise within the field of neuroscience, as scientists with firsthand experience of the condition are uniquely positioned to approach the problem with empathy and urgency.
This coupling of personal experience and scientific inquiry has transformed the dynamics of their research efforts, emphasizing the importance of collaboration in addressing complex health challenges. Vallabh and Minikel’s lab is staffed with a diverse team committed to advancing the understanding of prion pathophysiology and exploring potential gene-editing therapies. Their collective efforts demonstrate that the scientific community can indeed come together to drive progress in treating diseases that have long remained enigmatic. Such collaborations not only enrich research endeavors but also foster a greater sense of shared purpose among scientists and patients alike.
The Future of Prion Disease Research: Challenges and Opportunities
As researchers embark on the journey toward developing effective treatments for prion disease, the path forward is fraught with both challenges and opportunities. Although significant progress has been made in understanding the mechanisms behind diseases like Creutzfeldt-Jakob disease and fatal familial insomnia, there remains a crucial need for more extensive research. Challenges include navigating the complexities of human prion protein testing, which requires balancing safety with the pressing need for advancements. Despite setbacks, scientists continue to innovate and refine their methodologies, ensuring that they are prepared to tackle any obstacles that may arise.
Additionally, the rapid evolution of gene-editing technologies provides a promising avenue for overcoming these challenges. Innovations in techniques like CRISPR and base editing are paving the way for unprecedented possibilities in treating genetic conditions, including prion diseases. Researchers are eager to explore how these cutting-edge methods can be harnessed to develop targeted therapies that might one day eliminate the impact of prion disorders on affected families.
In this ever-evolving landscape of prion disease research, the importance of interdisciplinary collaboration cannot be overstated. As scientists from various fields—including genetics, neuroscience, and bioengineering—come together, they bring different perspectives and expertise to the table. This collaborative environment fosters creative problem-solving and accelerates the development of potential therapies while ensuring that patient safety remains a top priority. The future of prion disease treatment lies in the collaborative spirit of researchers dedicated to understanding the underlying mechanisms of these disorders and translating their findings into clinical applications.
Ethical Considerations in Prion Disease Research
With the promising developments in prion disease treatment through gene editing, ethical considerations have become increasingly important. As researchers rush to advance their findings from the laboratory to clinical applications, they must account for the potential implications and consequences of their work. One significant concern is the ethical treatment of patients involved in clinical trials, particularly those with inherited mutations in the prion protein gene. It is imperative to ensure that participants are fully informed about the risks and benefits associated with experimental therapies, as well as maintaining rigorous standards for consent and safety throughout the research process.
Additionally, the potential for gene-editing techniques to be misused raises questions about the long-term effects of altering human DNA. Maintaining a transparent dialogue with the public and engaging with bioethicists and regulatory bodies will be crucial in addressing these concerns and ensuring that research follows ethical guidelines. As the field of prion disease research moves forward, it is essential to balance scientific innovation with ethical responsibility to gain public trust and support.
Furthermore, exploring the implications of genetic interventions leads to broader discussions about genetic privacy and the potential for discrimination based on an individual’s genetic makeup. It is crucial for researchers and policymakers to ensure that advancements in gene editing do not lead to stigma or unfair treatment of individuals with genetic disorders like prion diseases. The conversation around ethics in prion disease research should include input from affected patients, advocacy groups, and ethicists alike, fostering a collaborative effort to navigate these complex issues. Ultimately, engaging in ethical reflection will strengthen the foundation upon which future prion disease treatments are built.
Frequently Asked Questions
What are the latest advancements in prion disease treatment through gene-editing therapy?
Recent advancements in prion disease treatment include groundbreaking gene-editing therapy that targets the prion protein gene. This research has shown the potential to reduce toxic prion protein levels and prolong survival in mouse models, paving the way for future human trials.
How do researchers approach the development of treatments for Creutzfeldt-Jakob disease?
Researchers are exploring innovative treatments for Creutzfeldt-Jakob disease by using gene-editing therapies aimed at the prion protein gene. Studies indicate that modifying specific genetic elements can significantly lower the levels of harmful proteins in affected brains, offering hope for effective treatments.
What role does neuroscience research play in finding a treatment for fatal familial insomnia?
Neuroscience research is crucial in the quest for treatment for fatal familial insomnia as it helps scientists understand the mechanisms of prion diseases. New insights into prion protein gene mutations and their effects on brain function are driving the development of gene-editing therapies aimed at halting disease progression.
Is there hope for a cure for prion diseases like fatal familial insomnia and Creutzfeldt-Jakob disease?
While a definitive cure for prion diseases like fatal familial insomnia and Creutzfeldt-Jakob disease is still under investigation, emerging gene-editing therapies provide promising tools to potentially treat these conditions by targeting the root causes at the genetic level.
What are the implications of recent studies on prion disease treatment for future clinical trials?
Recent studies on prion disease treatment have significant implications for future clinical trials as they validate the potential of gene-editing therapies. Researchers are optimistic that forthcoming trials could lead to effective treatments for conditions like Creutzfeldt-Jakob disease, although many regulatory and technical hurdles remain.
How does the collaboration between patient-scientists influence prion disease research?
Collaboration between patient-scientists, like Sonia Vallabh and Eric Minikel, profoundly influences prion disease research by infusing personal motivation and unique perspectives into the work. Their experiences guide research priorities and enhance the relevance of gene-editing therapies being developed.
What challenges do researchers face in prion disease treatment development?
Researchers face several challenges in prion disease treatment development, including ensuring the safety of gene-editing therapies, optimizing delivery mechanisms, and addressing the complexities of prion diseases. The intricate nature of prion proteins demands innovative solutions to achieve effective therapies.
How does gene-editing therapy work in the context of prion diseases?
Gene-editing therapy in the context of prion diseases works by specifically altering the genetic sequences that produce abnormal prion proteins. This technique targets the prion protein gene, aiming to reduce the production of harmful proteins associated with conditions like Creutzfeldt-Jakob disease.
| Key Point | Details |
|---|---|
| Gene-Editing Therapy | A promising gene-editing therapy has been developed to treat prion disease, aiming to reduce misfolded proteins in the brain. |
| Study Findings | Research showed that altering a single base in the gene can reduce harmful protein levels by 50% in mice, extending lifespans by up to 52%. |
| Prion Diseases | Includes disorders like Creutzfeldt-Jakob disease and fatal familial insomnia, caused by abnormal protein folding. |
| Personal Connection | Sonia Vallabh has inherited fatal familial insomnia, motivating her and her husband, Eric Minikel, to pursue treatment research. |
| Collaborative Efforts | Research involves cross-disciplinary collaboration, integrating expertise from different labs to enhance treatment potential. |
| Future Trials | Human trials are not expected for several years, requiring further refinement of the gene-editing technique. |
Summary
Prion disease treatment is moving towards a significant breakthrough, thanks to advancing research in gene-editing therapies. A recent study highlights how altering specific genes can drastically reduce the presence of harmful proteins that severely affect brain function. The personal story of researchers like Sonia Vallabh adds a profound narrative to this scientific journey, showing that not only are they working to find a cure, but they are also motivated by their personal experiences with the disease. As we await the next steps towards human trials, the collaborative efforts in this field indicate a promising future for those affected by prion diseases.