Gene Therapy for Hemophilia: A Life-Changing Breakthrough
Gene therapy for hemophilia marks a revolutionary advancement in the treatment landscape for this inherited bleeding disorder, offering hope where traditional management has fallen short. Patients like Terence Blue, who recently became the first patient in New England to receive the FDA-approved therapy Hemgenix, are already experiencing remarkable benefits, including reduced reliance on frequent factor injections. With the potential to provide long-lasting correction of the underlying genetic defect, gene therapy for hemophilia B offers a promising glimpse into the future of hemophilia treatment. As researchers continue to explore the gene therapy benefits, the excitement surrounds the prospect of a true hemophilia B cure that could transform the lives of those affected. This innovative approach highlights the thrilling advancements being made as genetic research moves from labs to real-world applications, paving the way for safer and more effective therapies.
The innovative realm of genetic medicine is reshaping the treatment approaches for hemophilia, positioning itself as a beacon of hope for patients with this bleeding disorder. By utilizing advanced techniques to alter the genetic root causes, this form of therapy aims to alleviate the burdensome life-long dependence on clotting factor injections. Known colloquially as gene therapy, this transformative treatment offers an opportunity for patients to break free from the constraints of their condition. As evidenced by the experiences of individuals like Terence Blue, who received the groundbreaking Hemgenix, the potential outcomes are not only life-changing but also hint at a future where hemophilia could potentially be eradicated. With continuous research and development, the field is moving towards solutions that harness the body’s own biological machinery to correct inherited genetic anomalies, thereby promising a brighter future for those affected by hemophilia.
Exploring Gene Therapy for Hemophilia B
Gene therapy represents a revolutionary approach in the treatment of hemophilia B, aiming to alleviate the lifelong burden of care for patients like Terence Blue. With therapies like Hemgenix, patients can potentially experience significant improvements in their quality of life, freeing them from the daily grind of injections. This innovative approach works by introducing corrected copies of genes into patients’ cells, effectively addressing the underlying cause of the disease rather than just treating its symptoms. As gene therapy continues to evolve, it holds the promise of transforming hemophilia treatment from a chronic condition into a manageable one.
The benefits of gene therapy extend beyond immediate symptom relief. Data from clinical trials indicate that a significant majority of patients treated with Hemgenix do not require regular factor IX infusions, indicating a long-lasting impact on their condition. This not only translates to reduced healthcare costs in the long term but also allows individuals to engage more fully in life without the constant fear of bleeding episodes. As such, gene therapy represents not just a treatment but a potential cure for hemophilia B, opening a new avenue towards improved health outcomes for patients.
The Impact of Hemgenix on Patient Lives
Hemgenix has emerged as a groundbreaking treatment in the realm of hemophilia therapy, particularly for those diagnosed with hemophilia B. Patients like Terence Blue have reported remarkable improvements following their treatments, describing their experiences as life-changing. The ability to conduct daily activities without the fear of sudden bleeding incidents signifies a profound change in the way patients manage their lives. This therapy not only enhances physical healing but also significantly boosts the emotional well-being of patients as they gain independence from their previous regimented care routines.
Beyond individual stories, the wider implications of Hemgenix for hemophilia treatment are substantial. As the FDA approved this gene therapy, it paved the way for more options that could enhance the overall management of hemophilia. With ongoing advancements in biotechnology, the hope is to develop more therapies like Hemgenix that can offer sustained relief and improved quality of life for patients. Each success story in gene therapy adds to the foundation of trust in these innovative treatments, inspiring further research and development in the field.
Navigating Market Pressures in Gene Therapy
The introduction of gene therapies like Hemgenix comes with significant market challenges. Despite their revolutionary potential, these treatments often face scrutiny over their pricing and accessibility. With costs reaching into the millions, as seen with Hemgenix, healthcare systems are tested as they try to balance innovative treatments with economic viability. Patient acceptance and market readiness play crucial roles in determining whether these therapies can consistently reach those who need them. High prices can deter patients and insurance providers, leading to complications in the therapy’s rollout and sustained availability.
Market pressures have already influenced the fate of other gene therapies targeting hemophilia and similar conditions. The withdrawal of therapies from the market due to limited patient interest underscores the importance of addressing both clinical effectiveness and economic realities. As healthcare providers and policymakers work toward solutions, understanding the balance between groundbreaking treatments and their affordability will be essential in ensuring that advancements like Hemgenix truly benefit those in need.
Long-Term Benefits of Gene Therapy in Hemophilia Management
While immediate benefits of gene therapy such as Hemgenix are being celebrated, the long-term prospects are equally promising. Patients who receive such therapies often find themselves with a reduced need for ongoing treatment, promoting a better quality of life. In the case of Hemgenix, clinical studies have shown that a significant percentage of patients maintain adequate factor IX levels years after treatment, indicating a durable response that may resemble a cure rather than a mere therapy.
The ability to achieve sustained factor IX activity could change the landscape of hemophilia management forever. Individuals who previously relied on frequent infusions may find themselves able to lead more active and engaged lives, free from the regular hospital visits that dominated their daily routines. As more data becomes available regarding long-term outcomes, healthcare providers can better inform patients about the tangible benefits gene therapies might bring, ultimately fostering a new era in the management of hemophilia.
The Future of Gene Therapy and Hemophilia Treatment
Looking ahead, the future developments in gene therapy for hemophilia present an exciting frontier in medical science. With the successful introduction of Hemgenix, researchers are motivated to explore additional gene therapies that could address other types of hemophilia and genetic disorders. The potential to further refine these therapies could lead to even greater efficacy, safety, and access for patients, transforming the standard of care in hematology.
However, as we innovate, a balanced focus on ethical considerations and patient education will be necessary to ensure safe practice. Initiatives to inform patients about the ways gene therapy can reshape their lives will be essential, alongside dialogues about regulatory frameworks that can support these therapies without compromising patient safety. As the science progresses, the commitment to patient-centered care will ensure that therapies like Hemgenix can fulfill their promise across diverse populations.
Clinical Considerations in Adopting Gene Therapies
The integration of gene therapy into clinical practice requires careful consideration of patient selection and treatment protocols. As evidenced by Terence Blue’s journey, the pathway to receiving Hemgenix involves extensive evaluation and monitoring. The therapy must be chosen based on individual health circumstances and needs, highlighting the importance of personalized medicine in treating hemophilia. Healthcare providers must stay informed about the evolving landscape of gene therapies to make the best recommendations for their patients.
Adoption of gene therapy also brings with it a need for ongoing patient support and education. Patients may have concerns about the long-term efficacy and any potential risks associated with introducing novel gene treatments. Establishing open lines of communication can help mitigate fears while instilling confidence in the treatment plan. Alongside the clinical data, sharing success stories from other patients can empower individuals to embrace cutting-edge therapies that have the potential to significantly improve their lives.
Living Life Beyond Hemophilia: Patient Perspectives
Patients living with hemophilia often face unique challenges that extend beyond mere medical treatment. Terence Blue’s story is a poignant illustration of the emotional strains that accompany chronic health conditions. The prospect of gene therapy like Hemgenix gives hope not only for clinical improvements but also for enhanced social interactions and emotional well-being. Individuals report feeling liberated from the burdens of constant medication management, allowing them to engage more fully in everyday life.
Sharing the experiences of patients who have undergone gene therapy is critical in forming a supportive community. Connection among patients can foster a sense of belonging and shared understanding, where stories of healing and triumph resonate deeply. As conversations around gene therapy grow, so too does the opportunity for patients to inspire one another, building a network of encouragement and resilience.
Ethical Implications of Gene Therapy in Hemophilia
As the field of gene therapy advances, ethical considerations become paramount. The potential for life-changing treatments like Hemgenix raises essential questions about accessibility, equity, and informed consent. Healthcare systems must ensure that all patients, regardless of socioeconomic status, have equal access to these innovative therapies. Safety, efficacy, and monitoring must also be prioritized to uphold ethical standards in the application of gene therapies.
Moreover, discussions around gene therapy provoke important dialogues about the long-term implications of genetic modifications. Patients and providers need to explore the unknown risks versus the potential benefits, ensuring transparent communication throughout the treatment process. By balancing innovation with ethical responsibility, the medical community can help pave the way for the responsible integration of gene therapy into standard hemophilia care.
The Role of Research in Advancing Gene Therapies
The ongoing research into gene therapies for hemophilia is crucial to developing effective treatments that can redefine patient experiences. Clinical trials for therapies like Hemgenix provide critical data that fuels scientific understanding and guides future innovations. The learning derived from each new study not only shapes treatment protocols but also deepens the medical community’s grasp of hemophilia’s complexities and long-term management strategies.
Advancements in technology and biotechnology play a pivotal role in improving gene therapy outcomes. Continued investment in research allows for exploration of new delivery mechanisms and targeted approaches that can lead to better therapeutic results. As researchers uncover more about the genetic basis of hemophilia, the potential grows for novel therapies that could one day make living with hemophilia a thing of the past, offering hope to patients and families alike.
Frequently Asked Questions
What is gene therapy for hemophilia and how does it work?
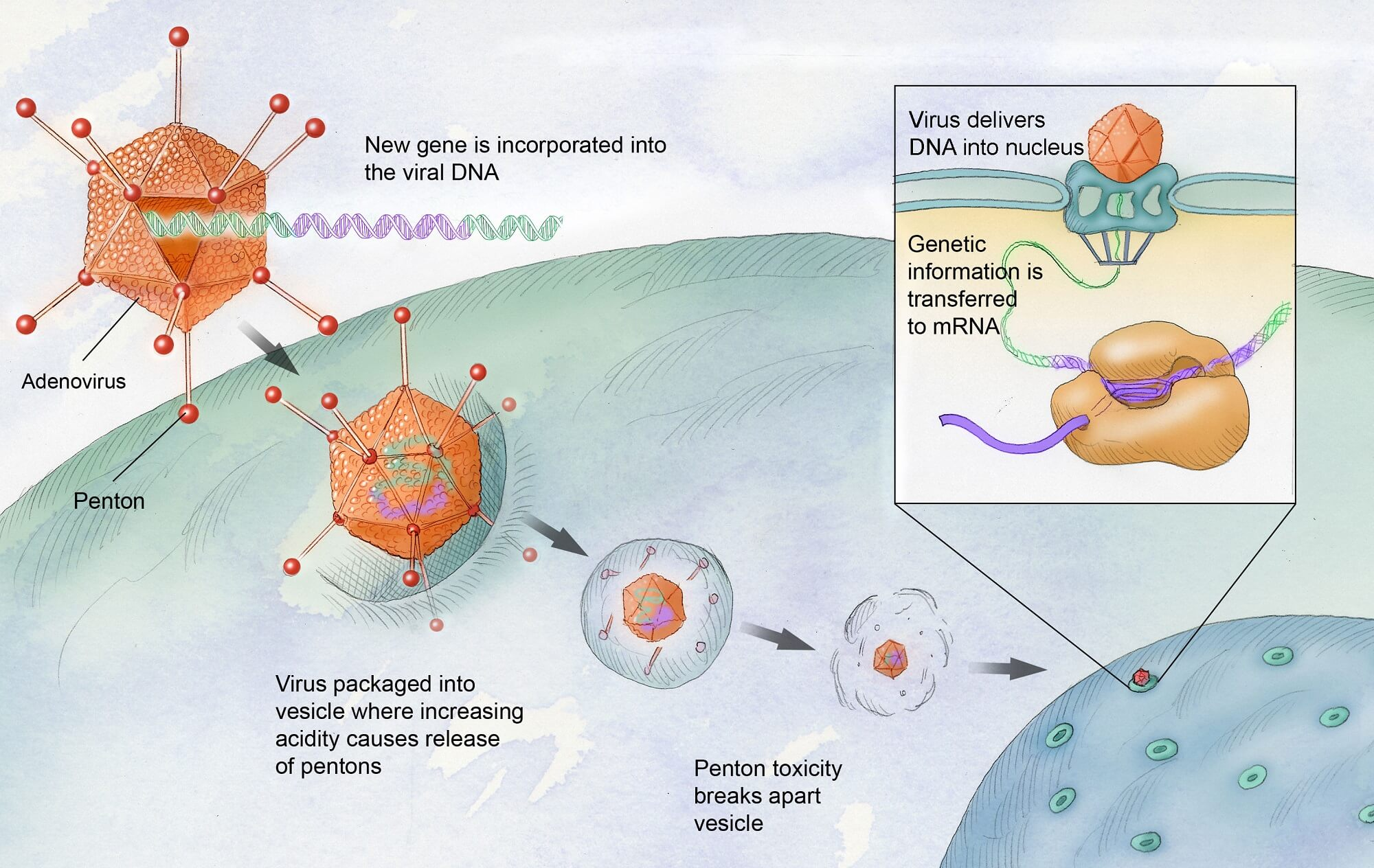
Gene therapy for hemophilia involves introducing a normal copy of the gene responsible for producing clotting factor into the patient’s body. In the case of hemophilia B, a virus is used to deliver this gene to liver cells, allowing them to produce the clotting factor IX that is deficient or missing in hemophilia patients. This innovative treatment aims to reduce or eliminate the need for regular clotting factor injections.
What are the benefits of gene therapy for hemophilia treatment?
The benefits of gene therapy for hemophilia treatment include the potential for long-lasting correction of the clotting factor deficiency, significant reduction in the frequency of bleeding episodes, and decreased dependence on factor replacement therapies. Patients, like Terence Blue, have reported healing faster and having a better quality of life following gene therapy like Hemgenix.
How is Hemgenix approved for hemophilia B and what is its significance?
Hemgenix is a gene therapy approved by the FDA specifically for hemophilia B. It represents a significant breakthrough as it’s designed to address the underlying genetic cause of the disease rather than just managing symptoms. This approval marks a critical advancement in the field of gene therapies, demonstrating the potential for long-term solutions for patients with hemophilia.
What can be expected during and after the administration of gene therapy for hemophilia?
During the administration of gene therapy for hemophilia, patients typically undergo an outpatient infusion that lasts a couple of hours. Following the infusion, they are monitored for any side effects. Post-treatment, patients may experience an increase in clotting factor levels, as seen in Terence Blue, who observed a rise from less than 1% to 32%. Most patients report improvements in bleeding control and overall health.
Are there any side effects associated with gene therapy for hemophilia?
Like any medical treatment, gene therapy for hemophilia can have side effects. Common side effects may include reactions to the infusion, elevated liver enzymes, or flu-like symptoms. However, many patients tolerate the treatment well and report minimal side effects. Monitoring by healthcare professionals ensures any complications are managed promptly.
Is gene therapy a cure for hemophilia or just a treatment option?
While gene therapy, such as Hemgenix, shows promise and may lead to long-lasting effects, it is currently described as a treatment rather than a definitive cure. Many patients do not require factor IX prophylaxis after therapy, suggesting a significant improvement in their condition. Long-term studies are necessary to fully assess the durability of the benefits provided by gene therapy for hemophilia.
What makes gene therapy for hemophilia different from traditional treatments?
Gene therapy for hemophilia differs from traditional treatments by targeting the root cause of the disease rather than just managing symptoms. Traditional treatments involve regular injections of clotting factors to prevent bleeding. In contrast, gene therapy aims to correct the genetic defect causing the deficiency, potentially freeing patients from frequent treatments and improving their long-term outcomes.
What is the future of gene therapy in hemophilia care?
The future of gene therapy in hemophilia care looks promising as ongoing research develops new therapies and refines existing ones. There’s optimism within the medical community that gene therapies will provide effective, long-lasting solutions for hemophilia patients. Market dynamics, patient acceptance, and affordability will shape the broader use of these innovative therapies in clinical practice.
| Key Point | Description |
|---|---|
| Terence Blue’s Experience | The first patient in New England to receive gene therapy for hemophilia B at Brigham and Women’s Hospital. |
| What is Hemgenix? | A gene therapy approved by the FDA in November 2022, designed to produce clotting factor IX in patients. |
| Significance of Gene Therapy | Offers a potential long-term solution for hemophilia, reducing the need for constant injections. |
| Challenges Facing Gene Therapy | High treatment costs and market pressures can hinder the availability of treatments. |
| Clinical Success Rate | 94% of patients treated with Hemgenix in trials do not require factor IX prophylaxis three years later. |
| Personal Changes Post-Treatment | Terence has noticed significant improvement in healing and reduced need for factor treatment. |
Summary
Gene therapy for hemophilia offers groundbreaking prospects for patients like Terence Blue, marking a significant advancement in treatment options. By directly addressing the genetic basis of hemophilia B, Hemgenix presents a transformative approach that could alleviate years of dependency on regular factor IX injections. This innovative treatment not only promises to enhance the quality of life but also raises important discussions about the economics of gene therapies and their accessibility. As research continues, the future of hemophilia treatment looks increasingly hopeful.